
Understanding CLCN4-NDC Using Rat Models
This article provides a family-friendly summary of one of the projects funded by the recent Cure CLCN4 Pilot Grant. It explains, in clear and accessible language, what the research aimed to do, what the scientists found, and why these results matter for individuals and families living with CLCN4-related neurodevelopmental condition (CLCN4-NDC).
Project details
Project supported by the Cure CLCN4 Pilot Grant Programme
Researchers: Dr Yann Herault & Dr Tania Sorg (IGMCB, France)
Project period: April 2024 – October 2025
What was the project about?
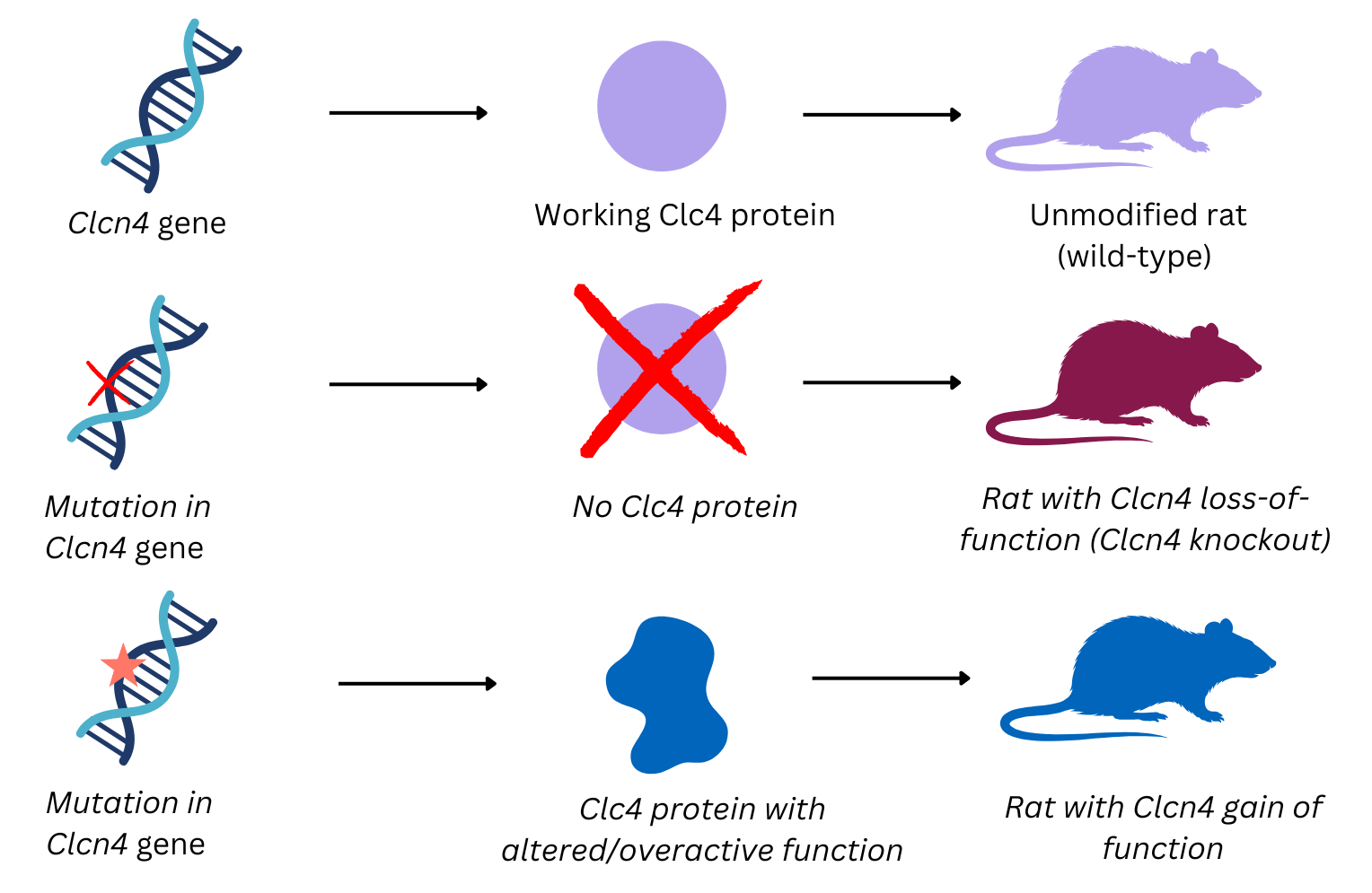
With support from the Cure CLCN4 Pilot Grant, Dr Yann Herault and Dr Tania Sorg studied the recently generated rat models, carrying changes in the Clcn4 gene. Their goal was to understand if these genetic changes lead to the features seen in people with CLCN4-NDC.
People with CLCN4-NDC can have different types of genetic changes in the Clcn4 gene. In some individuals, the gene becomes overactive, while in others it is partially or completely switched off. To reflect this important difference seen in families, the researchers studied two different rat models:
- A gain-of-function (GOF) model (A549V), in which the ClC4 protein is overactive, similar to what happens in some people with pathogenic CLCN4 variants.
- A loss-of-function (LOF) model, in which the Clcn4 gene is switched off, meaning no ClC4 protein is produced, as seen in other individuals with CLCN4-NDC.
Both models were studied in detail to see if and how these genetic changes affected learning, behaviour, movement, growth, and overall health.
Key findings
The A549V gain-of-function model reproduced important features of the human condition, especially in male rats:
- difficulties with learning and memory tasks,
- hyperactivity and stress sensitivity,
- reduced muscle strength and coordination,
- lower body weight and food intake,
Female A549V rats showed only mild changes. In contrast, rats missing the Clcn4 gene entirely (loss-of-function) looked mostly normal, suggesting that a related gene, Clcn3, may compensate for the loss of Clcn4.
This makes the A549V rat a valuable disease model for studying how Clcn4 changes affect the brain and for testing future treatments.
Why this matters
This is the first time CLCN4 variants have been studied in rats. Unlike mice, the new A549V gain-of-function rat model, mainly males, mirrors some of the symptoms seen in humans, offering scientists a better tool to:
- explore how pathogenic CLCN4 variants affect brain circuits and behaviour,
- test new therapies in a controlled way
What happens next
The researchers now want to repeat the study with more animals and additional assays to make sure the results are strong and reliable. They will also look more closely at how stress affects symptoms, and explore whether other related genes might explain why the loss-of-function rats did not show many changes.
Both CLCN4 rat lines, plus a third line that has not yet been studied, are now freely available to the scientific community through the INFRAFRONTIER repository:
Thank you
Dr Herault and Dr Sorg’s work shows how small pilot grants can lead to major progress in understanding rare conditions like CLCN4-NDC. Their findings will help guide future research, refine animal models, and, ultimately, bring us closer to effective treatments for families worldwide.

