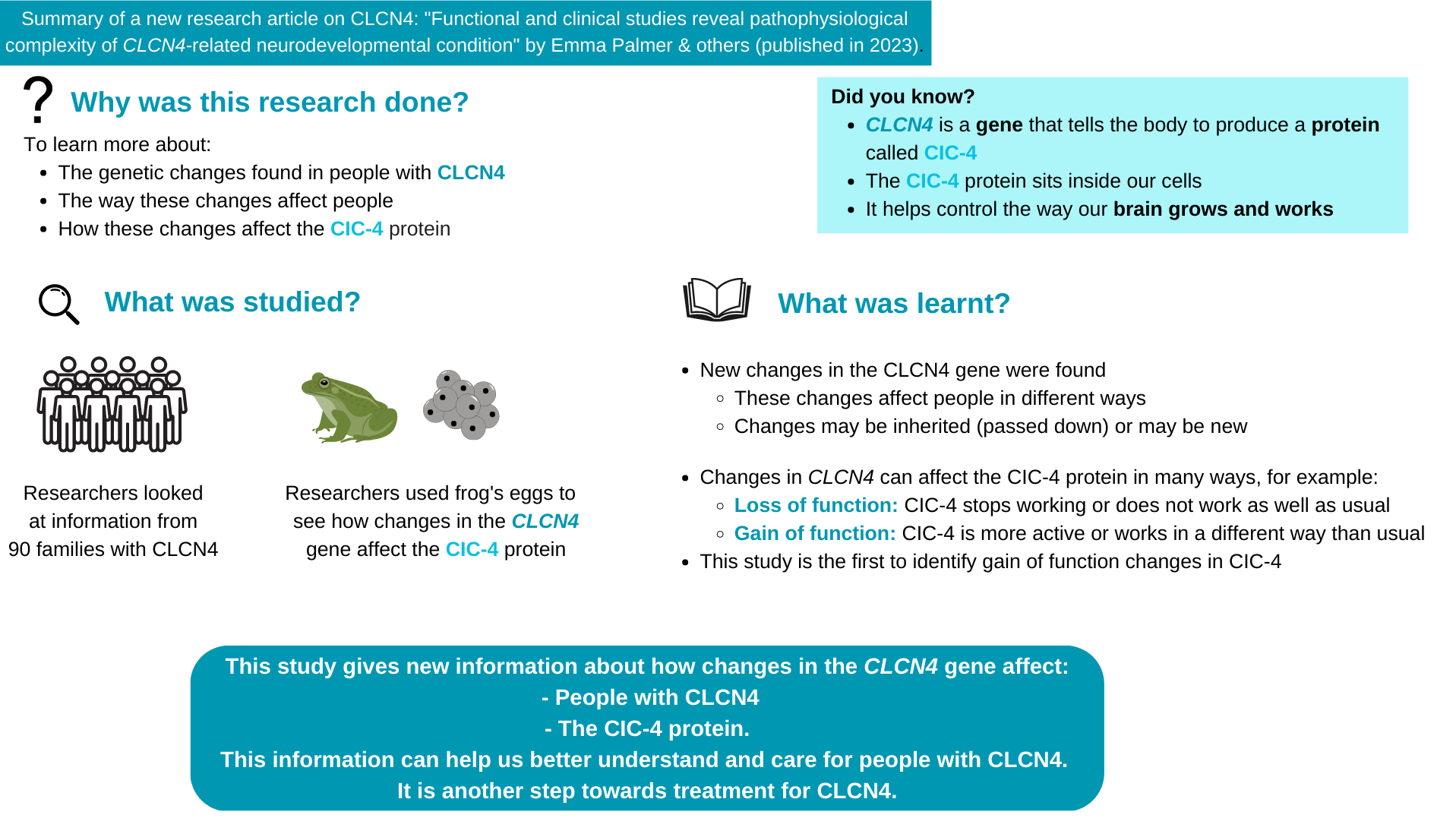
Palmer et al. 2023 – A Summary for Families
Do you or a loved one have a diagnosis of CLCN4-related condition? Stay informed on the latest research with our family-friendly summary of the most recent scientific paper titled “Functional and clinical studies reveal pathophysiological complexity of CLCN4-related neurodevelopmental condition” by Palmer et al. 2023. This summary breaks down the key findings and implications of the research in an easy-to-digest format so that anyone can understand this latest advancement. Looking for an even shorter description? Click here or scroll to the end of this page for a great snapshot summary!

Key reminder points
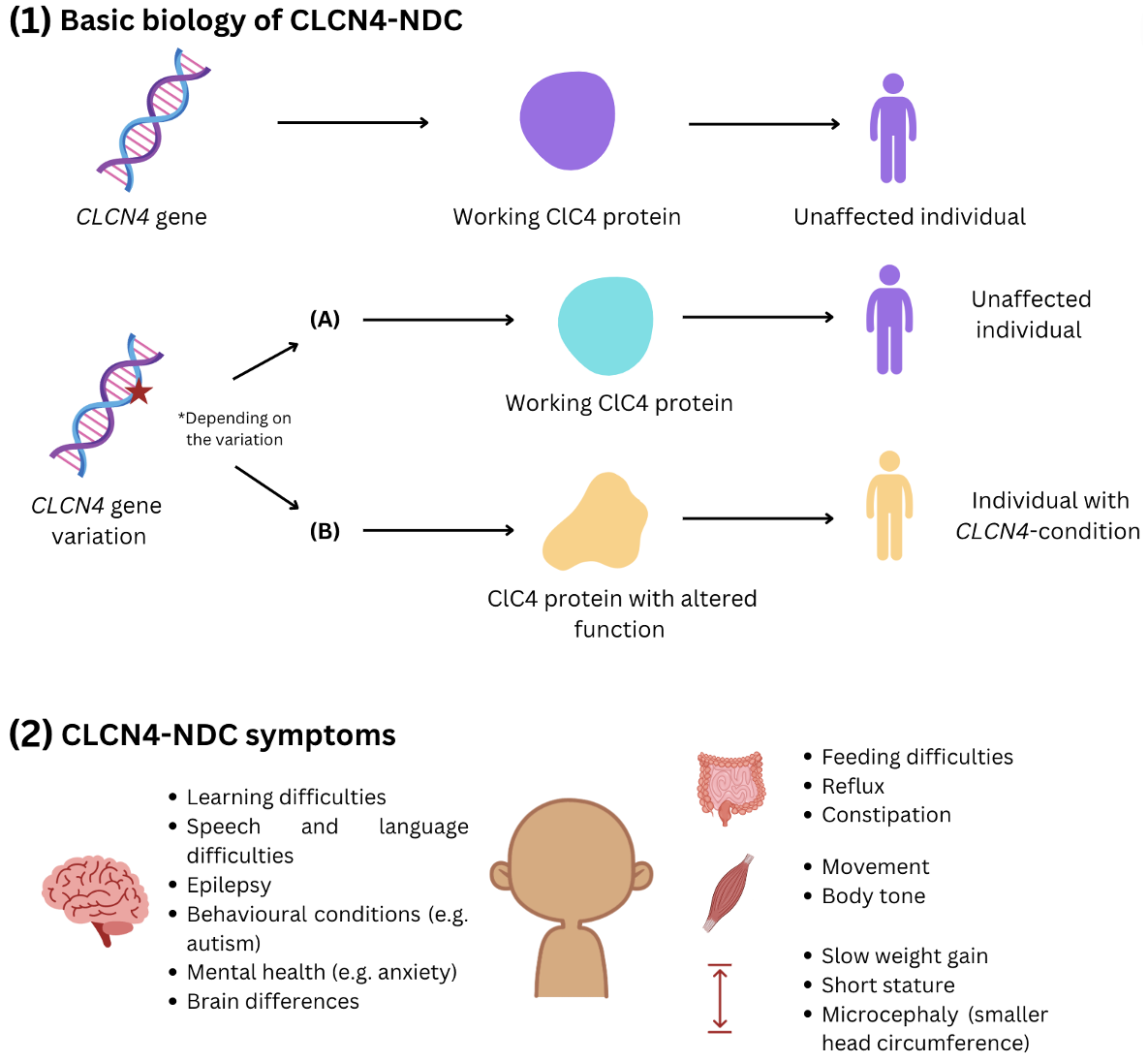
- CLCN4-related neurodevelopmental condition (CLCN4-NDC) is caused by variants or ‘changes’ in the CLCN4 gene, which located on the X chromosome.
- The CLCN4 gene gives instructions to make a protein called ClC-4.
- Some changes in the CLCN4 gene affect how the ClC-4 protein works inside the cells of an affected individual’s body.
- It is thought that this protein has important roles in brain and nerve cell function.
- However, we still have more to learn about all the roles of the ClC-4 protein.
- So far, the main symptoms of CLCN4-NDC impact the neurological system. They can include learning difficulties, epilepsy, behavioural conditions including autism, and differences in the control of movement and body tone.
- Some individuals with CLCN4-NDC have other health conditions, including difficulties with feeding, reflux, constipation and challenges putting on weight.
What was done before
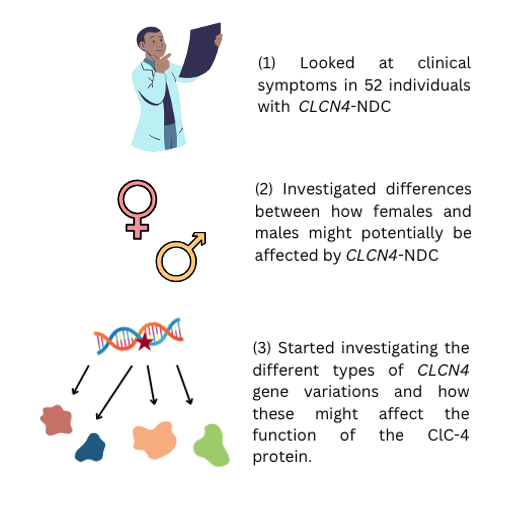
In 2018, Dr. Emma Palmer, Dr. Vera Kalscheuer and colleagues provided a description of the genetic and clinical characteristics of 52 individuals with CLCN4-NDC. Their findings are summarised below:
-
Main clinical features of individuals with CLCN4-NDC are intellectual disability, behavioural and psychiatric conditions and epilepsy. Brain white matter changes and differences in movement control (movement disorders) can also occur.
-
The impact on females seems to vary according to whether they are the first person in the family to have the CLCN4 genetic change (de novo), or whether they inherited the genetic change from a parent, with those with a de novo genetic change appearing to be more severely affected.
-
All males with a CLCN4 genetic change develop some symptoms but the number and severity of these symptoms varies between individuals.
-
They described the different ‘types’ of CLCN4 gene variants and started investigating if and how these variants can affect the function of the ClC-4 protein.
This work was the stepping-stone for the work presented in the new publication, Palmer et al. 2023, summarized below:
Aims of the new paper…Palmer et al. 2022
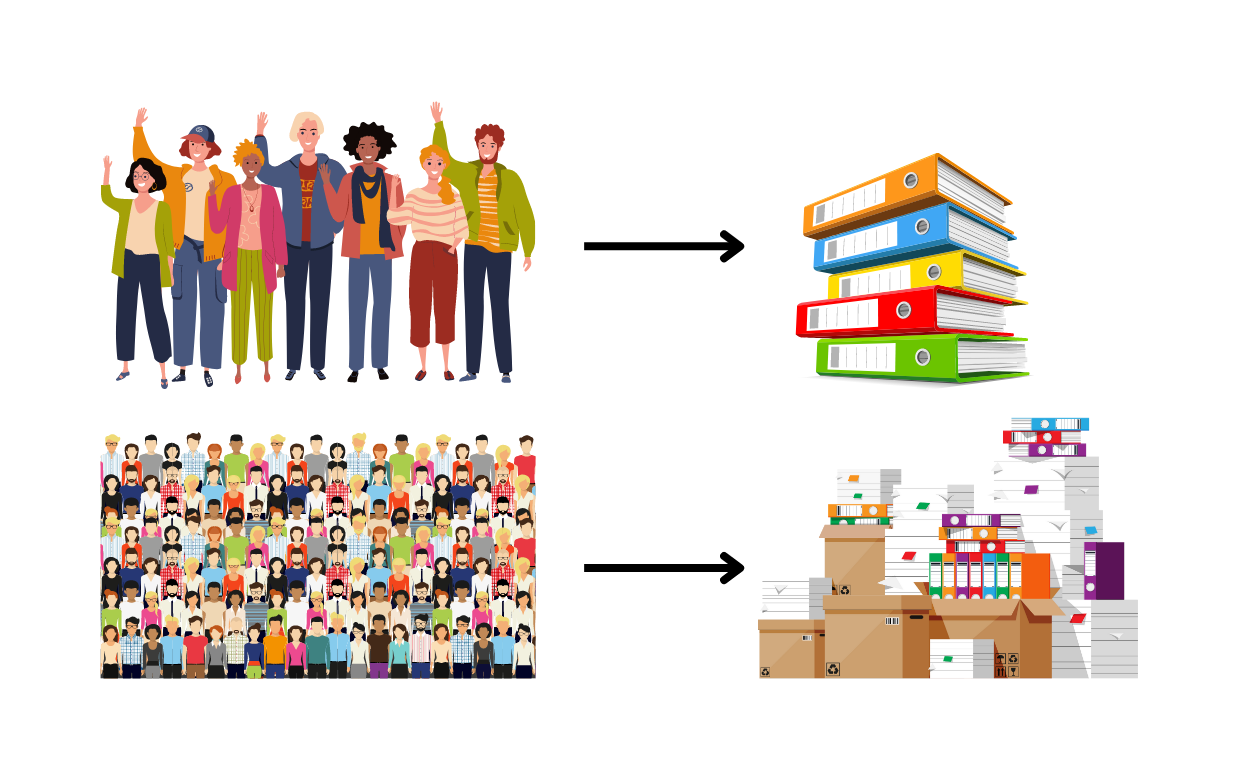
Aim 1. Investigate the genetic and clinical characteristics of a larger group of individuals with CLCN4 variants.
Why is this important?
- There is power in numbers! By looking at a larger number of patients we can get a better, more representative picture of the condition, its features and how the symptoms of the condition may change over time.
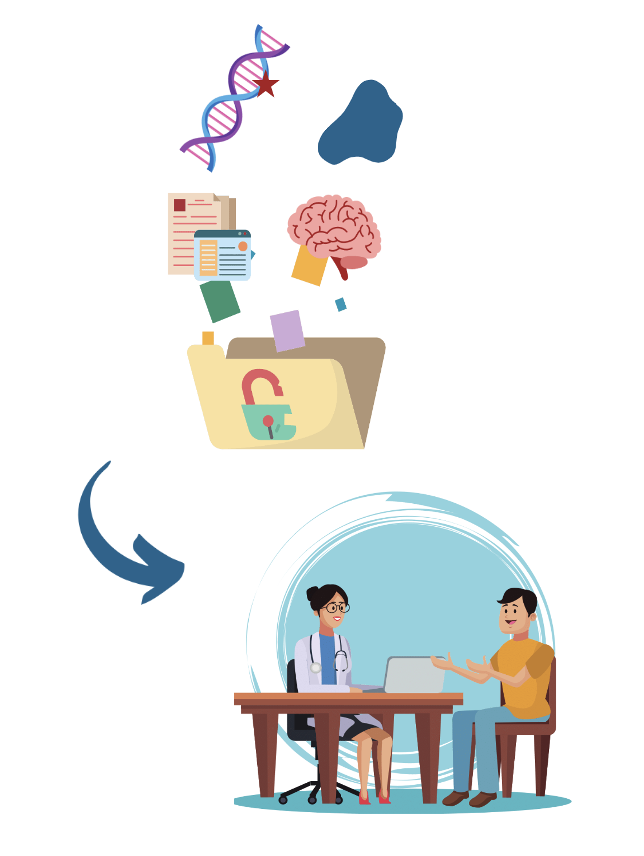
Aim 2. Investigate the range of CLCN4 gene variants, their effect on protein function, and how they link to clinical findings in patients.
Why is this important?
- Not all CLCN4 gene variants lead to CLCN4-NDC. There are many healthy unaffected individuals with variants in the CLCN4 gene whose ClC-4 protein works just fine.
- Understanding which genetic variants impact the function of the ClC-4 protein to cause the condition is essential in order to provide families with accurate information about the implications of the CLCN4 gene change that they or their loved ones carry.
- Different CLCN4 gene variants can have different effects on the function of ClC-4 protein. Investigating links between specific gene changes, their effects on the ClC-4 protein and how these relate to the presence and severity of symptoms will help provide important information to affected families and facilitate the development of potential therapies.
Overall, in the new publication, the authors aimed to expand on their previous work but looking at a larger cohort of individuals with CLCN4 gene changes. Through a collaborative and international effort with clinicians from all over the world they provided clinical data on 56 new families, and through a review of new and previously published cases they summarized features on 122 individuals with CLCN4 gene changes, the largest cohort to date. Finally, they looked at the effects of 59 different CLCN4 gene variants on ClC-4 function.
What they found
1. Summary of clinical features (*percentages given for total number reported cases; 56 new individuals + previously reported individuals)
Learning difficulties – are the most common clinical feature in males (most often males have a moderate to severe intellectual disability but sometimes learning difficulties are borderline or mild). In females, prediction of cognitive function is much harder. Individuals with de novo variants often have more significant learning difficulties, but that is not always the case.
Speech and language difficulties – 100% of males have delayed speech and language. Delayed speech and language affects the majority (95%) of females with de novo variants but less than 15% of females with inherited variants or variants where we do not yet know if they are inherited or de novo.
Behavioural and mental health conditions – the most common conditions are
- attention deficit hyperactivity disorder (ADHD/hyperactivity/impulsivity/restlessness (30% of all males; 35% females with a de novo variant);
- autism spectrum disorder. This was diagnosed in 25% of all males and 30% females with de novo variants;
- challenging behaviors/angry outbursts (28% males, 30% females with de novo variants);
- anxiety (17% of males; 45% females with de novo variants; 8.1% females with inherited variants/unknown inheritance).
Less frequent but also observed are obsessive compulsive behavior (10% males, 10% females with de novo variants), depression/bipolar disorder (8.6% males, 5% females with de novo variants).
Epilepsy – affects 60% of all males, 25% of females with de novo variants. The onset of epileptic seizures is typically within first 3 years of life (but there are a couple of individuals who developed in early teenage years). The most common type of seizures are generalized absence and tonic-clonic seizures and focal onset seizures. Epilepsy is often easy to treat with anti-seizure medications, but in some individuals can be harder and require multiple treatments. So far there is not a clear relationship between the severity of epilepsy and the severity of learning difficulties.
No specific anti-seizure medications have been so far shown to be more helpful in controlling seizures in individuals with CLCN4-NDC.
Brain differences – neuroimaging (MRI or CT scan of the brain) showed differences in the structure of the brain in just under 70% of males and 60% of females with de novo variants. The most common findings were changes to the brain white matter (57% males; 30% females with de novo variants) and abnormalities of the corpus callosum which is a white matter tract connecting the two halves of the brain (40% males; 23.5% females with de novo variants). None of these brain differences needed specific surgery or treatments.
Other neurological symptoms – infantile hypotonia (weak muscle tone) was reported in about 40% of males and 55% of females with de novo variants. Later onset neurological symptoms included tremor, ataxia (an unsteady gait), hyperkinesis (very rapid movements) or stereotypical (repetitive, unusual) movements, other changes in gait such as walking with a stooped posture, or progressive spasticity (increased tone), was observed in 26% of males and 40% of females with de novo variants.
Gastrointestinal issues – these affected many individuals. Symptoms included gastrointestinal reflux (15% males, 25% females with de novo variants); constipation (15% males, 35% females with de novo variants); feeding difficulties (17% males, 55% females with de novo variants).
Growth issues – Slow weight gain affected about 15 % males and 30% of females with de novo variants. Some individuals had short stature (15% males, 30% females with de novo variants). Progressive microcephaly (a smaller head circumference than typical) was more common in females with de novo variants (~70%) compared to males (~20%)
Other findings – other less commonly noted clinical features included scoliosis (a sway in the spine), pes planus (flat feet) and/or lax joints, sleep disorders, otitis media with effusions (middle ear infections), and strabismus (squints).
However, other organs, such as the heart, kidney and liver, do not seem to be affected in individuals with CLCN4-NDC. There was no evidence for an increased risk of cancers.
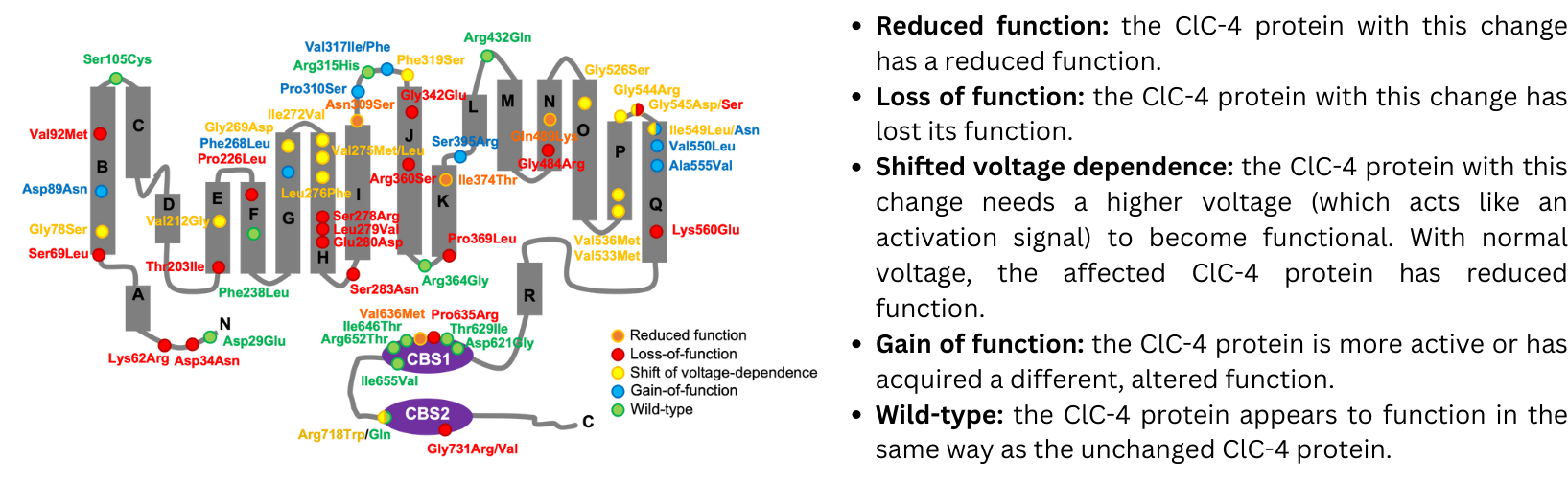
2. Summary of analysis of CLCN4 gene variants and their effect on ClC-4 protein function
The potential effects of the CLCN4 gene variants on the function of the ClC-4 protein were investigated using a technique that allows scientists to compare the function of the ‘changed’ ClC-4 found in patients with a normally functioning ClC-4. This method allows researchers not only to determine whether the changed ClC-4 protein is functioning differently, and also in what way (e.g. not working at all, working less, working in a different way). This is very critical knowledge for efforts to develop effective therapies for CLCN4-NDC.
The figure below, obtained from the new publication, shows a schematic of the ClC-4 protein (in grey), with each dot representing a variant in the ClC-4 protein found in 59 of the patients included in the study, and with the different colors indicating how that specific change has affected the function of the protein. Overall, the results revealed a wider variety of impacts on the function of ClC-4 than previously described, highlighting the complexity of CLCN4-NDC and providing key insights to guide further research efforts.
What are the implications of the findings?
Although further work is needed, the publication significantly expands our knowledge of the different CLCN4 gene variants that there are and how some of them affect the function of the ClC-4 protein. We now have summarised clinical information on a much larger group of individuals, 122 people, with CLCN4-NDC. This helps us better understand how CLCN4-NDC presents in diagnosed individuals, and provide updated management guidelines for clinicians (read them here).
What’s next?
More research! While this new publication has significantly increased our knowledge of CLCN4-NDC, further work is needed to continue learning about the spectrum of CLCN4 gene variants and their implications on protein function and clinical symptoms. The more we understand about the function of ClC-4 and CLCN4-NDC the more chances of finding effective therapeutic options.

Can I help?
Yes, you can! If you or a close family member have a diagnosis of CLCN4-NDC you can help us learn more about the condition by participating in the CLCN4 patient registry, hosted by Simons Searchlight. You can learn more about the registry and how to sign up here!
You can also help by donating or helping us fundraise so that we can keep supporting the much needed scientific research on CLCN4-related condition.
Thank you to the authors
On behalf of the CLCN4 community we would like to thank the authors of the paper, with a special mention to Dr. Emma Palmer, Dr. Vera Kalscheuer and Dr. Michael Pusch, who led the efforts and work that resulted in this publication and helped prepare this summary. A big thank you to Natalie Grainger (UNSW) for her fantastic work in producing the snapshot summary.
Corporate Foundation Sponsors

